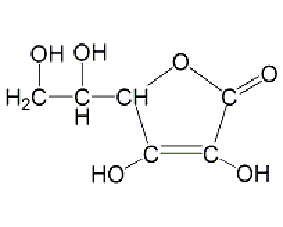
Structural formula
| Business number | 0147 |
|---|---|
| Molecular formula | C6H8O6 |
| Molecular weight | 176.12 |
| label |
Vitamin C, Vitamin C, ascorbic acid, 2,3,4,5,6-pentahydroxy-2-hexenoic acid-4-lactone, L-Threoascorbic acid, Antiscorbutic vitamins, Vitamin C, L-Ascorbic acid, 3-Oxo-L-gulofuranolactone, Antioxidants, food nutritional supplements, chromatographic analysis reagents, Ester solvent |
Numbering system
CAS number:50-81-7
MDL number:MFCD00064328
EINECS number:200-066-2
RTECS number:CI7650000
BRN number:84272
PubChem number:24891042
Physical property data
1. Character: Usually flake, sometimes needle-shaped monoclinic crystal. 2. Density (g/mL, 20/4℃): 1.954
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 191 (Microdecomposition)
5. Boiling point (ºC, normal pressure): 1.65
6. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1): -2339.8
7. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): -1164.6
8. Flash point (ºC): Undetermined
9. Specific rotation (º): [a] 25/D+20.5°-+21.5°(c=1)
10 . Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure ( KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in water , slightly soluble in ethanol, insoluble in chloroform, ether, benzene, petroleum ether, oils and fats.
Toxicological data
1. Acute toxicity: LD50: 5000mg/Kg (oral in rats)
Ecological data
None
Molecular structure data
1. Molar refractive index: 35.26
2. Molar volume (cm3/mol): 90.1
3. Isotonic specific volume (90.2K ): 310.3
4. Surface tension (dyne/cm): 140.5
5. Dielectric constant (F/m):
6. Dipole Distance (D):
7, Polarizability (10-24cm3): 13.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 8
6. Topological molecule polar surface area 107
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 232
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 2
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is relatively stable in dry air. Impure and many natural products can be oxidized by air and light. Its aqueous solution is unstable and quickly oxidizes to dehydroascorbic acid, especially in neutral or alkaline solutions. It is quickly oxidized and will accelerate oxidation when exposed to light, heat, metal ions such as iron and copper, and can form stable metal salts. It is a relatively strong reducing agent, and the color becomes darker after being stored for a long time, turning into varying degrees of light yellow. LC50 (mouse, intravenous): 518mg/kg.
2.Easily causes deterioration when exposed to air and heat. In alkaline solution It is prone to oxidation and failure. Under air conditions, it quickly deteriorates in aqueous solution and is a strong reducing agent.
3. Exists in flue gas.
4. Widely distributed in animals and plants. Stable to air when dry. Aqueous solutions are rapidly oxidized by air.
Storage method
Sealed packaging in brown glass bottles. Store in a cool, dry place away from light.
Synthesis method
1. Using glucose as raw material, it is hydrogenated under nickel catalysis to form sorbitol, which is then fermented and oxidized by Acetobacter to form L-sorbose, and then condensation reaction occurs with acetone under the catalysis of concentrated sulfuric acid to form diacetone L-sorbose. , and then oxidized to L-ascorbic acid with potassium permanganate under alkaline conditions. The production flow and technology are:
D-glucose reduction ↓ hydrogen, nickel fermentation oxidation ↓ acetobacter condensation ↓ acetone, sulfuric acid oxidation ↓ KMnO4
cyclization, deprotection ↓ HCl gas Body weight crystallization ↓ finished ethanol product
Catalytic hydrogenation of D-glucose using nickel as a catalyst can convert glucose into D-sorbitol. D-sorbitol is oxidized and fermented into L-sorbose under the action of Acetobacter, with a yield of more than 90%. Lsorbose is then separated by crystallization. This process can be carried out continuously and on a large scale.
Using sulfuric acid as a catalyst and treating L-sorbose with acetone, it can be converted into 2,3-O-isopropylidene-α-sorbose and 2,3,4,6-isopropylene. Mixture of propylene-α-L-sorbofuranosose. The reaction solution was neutralized, acetone was removed by distillation, and the product was extracted with toluene. Ferric chloride or ferric bromide can also be used instead of the catalyst sulfuric acid.
In dilute sodium hydroxide solution, sodium hypochlorite is used as the oxidant and nickel chloride is used as the catalyst to oxidize 2,3,4,6-isopropylideneαLsorbofuranos into 2,3,4,6-bis(O-isopropylidene)-2-oxo-L-gulonic acid. The yield can reach more than 90%. This oxidation reaction can also be oxidized with potassium permanganate under alkaline conditions, or directly electrochemically oxidized in an alkaline solution, or catalytically oxidized with oxygen in the presence of nickel or palladium.
There are several methods for removing the acetone protecting group and direct cyclization of 2,3,4,6-bis(O-isopropylidene)-2-oxo-L-gulonic acid. kind. One method is to treat gulonic acid with hydrogen chloride gas in a mixed solution of water, chloroform, and ethanol. At the end of the reaction, the product L-ascorbic acid can be filtered, with a yield greater than 80%, and then recrystallized with dilute ethanol to obtain the finished product of ascorbic acid.
Using glucose as raw material, it is oxidized to sorbitol under pressure under a nickel catalyst, and then fermented by Acetobacter and oxidized to L-sorbitol. It reacts with acetone under the catalysis of sulfuric acid to form diacetone-L-sorbitol, which is then oxidized to L-ascorbic acid by potassium permanganate under alkaline conditions.
D-sorbitol is made from glucose, and then oxidized and fermented to produce L-sorbose, which is condensed to form diacetone-L – Sorbose is then oxidized to form diacetone-2-keto-L-gluconic acid, which is then esterified into methyl 2-keto-L-gluconate, reacts with sodium methoxide to form sodium ascorbate, and is finally heated with hydrochloric acid to obtain ascorbic acid.
2.Usually, D-sorbitol can be made from glucose first, and then L-sorbose can be generated through oxidation and fermentation, and then Condensation generates diacetone-L-sorbose, which is then oxidized to generate diacetone-2-keto-L-ketogluconate, which is then esterified into methyl 2-keto-L-gluconate, and finally reacts with sodium methoxide to generate sodium ascorbate, which reacts with Hydrochloric acid is heated together to produce ascorbic acid.
Purpose
1. Can be used as nutritional supplement and antioxidant. Ascorbic acid is found in many foodsFor antioxidants, include processed fruits, vegetables, meat, fish, dried fruits, soft drinks and beverages. Added to pure fruit juice, it can maintain the flavor for a long time and strengthen vitamin C; added to cans and syrups, it can prevent peaches, apricots, cherries, etc. from changing color and taste when canned; added to beer and carbonated water, it can prevent oxidation and flavor deterioration. It can also be used as a wheat flour improver.
2.Reagents for the determination of arsenic, iron, iodine, bismuth, calcium, magnesium, titanium, tungsten, antimony and phosphorus. Reference material for acid anhydride determination. Also used as nutritional supplement and antioxidant.
3.Used as antioxidant and food nutrition fortifier. It can be used for fermented flour products, the maximum usage amount is 0.2g/kg; it can also be used for beer, the maximum usage amount is 0.04g/kg :Arial;”>g/kg.
4.Vitamin C participates in the body’s complex metabolic processes and can promote growth and enhance resistance to disease. This product has strong reducing properties and can be used as an antioxidant. When vitamin C is lacking, wounds or ulcers are not easy to heal, bones and teeth are easily broken, and scurvy symptoms occur: capillary permeability increases, the fragility increases, and it is easy to rupture and bleed; in severe cases, muscles and internal organs bleed, leading to death. Our country stipulates that it can be used to strengthen sandwich hard candies, and the usage amount is 2000~6000mg/kg; in high-speed iron cereals and their products (eat 50g of such foods per day) The dosage used in fortified infant foods is 800~1000mg/kg; the dosage used in fortified infant foods is 300~500 ;”>mg/kg; used in fortified canned fruits at a dosage of 200 to 400mg/kg; used in fortified liquids and milk drinks The dosage used in fortified fruit puree is 120~240mg/kg; the dosage used in fortified fruit puree is 50~100mg/kg “>mg/kg.
5.Vitamin C participates in the body’s complex metabolic processes, can promote growth and enhance resistance to disease, and can improve poultry production. Egg volume and improved egg shell quality. When animals are deficient in vitamin C, they will suffer from symptoms such as loss of appetite, growth stagnation, dull coat, and anemia.
6.Used as analytical reagents, such as reducing agents and masking agents. Used as chromatographic analysis reagents.
7.Used as an antioxidant in cosmetics and food to prevent pigmentation (old spots, freckles, chloasma) . The dosage in cosmetics is 0.1% to 0.5%, and the dosage in food is 0.005% to 0.5%.
8.Ascorbic acid is used in zinc-iron alloy plating, nickel-iron alloy electroplating solutions, precious metal chemical passivation solutions, and can also be used in solutions chemical analysis.
extended-reading:https://www.newtopchem.com/archives/40073extended-reading:https://www.bdmaee.net/dabco-t-1-catalyst-cas77-58-7-evonik-germany/extended-reading:https://www.cyclohexylamine.net/dimethylaminoethoxyethanol-cas-1704-62-7/extended-reading:https://www.bdmaee.net/pentamethyldipropene-triamine/extended-reading:https://www.cyclohexylamine.net/nn-dicyclohexylmethylamine-cas-7560-83-0-polycat-12/extended-reading:https://www.bdmaee.net/fomrez-ul-32-catalyst-bisdodecylthioctyltin-momentive-2/extended-reading:https://www.newtopchem.com/archives/1718extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/56.jpgextended-reading:https://www.bdmaee.net/low-odor-reactive-catalyst/extended-reading:https://www.newtopchem.com/archives/1105

