
Structural formula
| Business number | 018B |
|---|---|
| Molecular formula | C19H42BrN |
| Molecular weight | 364.46 |
| label |
Cetrimonium bromide, Cetyltrimethylammonium bromide, CTAB, Palmityltrimethylammonium bromide, Cetyltrimethylammonium bromide; cetyltrimethylammonium bromide, Bactericidal algaecide |
Numbering system
CAS number:57-09-0
MDL number:MFCD00011772
EINECS number:200-311-3
RTECS number:BQ7875000
BRN number:3598189
PubChem number:24895846
Physical property data
1. Properties: white microcrystalline powder. It is a quaternary ammonium salt. Hygroscopic. Stable in acidic solutions.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
>
4. Melting point (ºC): 237~243
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in 10 parts of water, easily soluble It is slightly soluble in acetone and almost insoluble in ether and benzene.
Toxicological data
1. Skin or eye irritation: mice, skin contact, open irritation test, 50mg/1H; rabbits, eye contact, standard Draize test, 450mg, strong reaction 2. Acute toxicity: rat oral LD50: 410mg/kg ; Rat intravenous LD50: 44mg/kg; Mouse intraperitoneal LC50: 106mg/kg; Mouse intravenous LD50: 32mg/kg; Rabbit intraperitoneal LD50: 125mg/kg; Rabbit subcutaneous LD50: 125mg/kg; Guinea pig subcutaneous LD50: 100mg/ kg3, other multiple dose toxicity: Oral TDLo in rats: 16380mg/kg/1Y-C4, Reproductive toxicity: Intraperitoneal TCLo in female mice: 10500ug/kg, conception after 8 days; Intraperitoneal TCLo in female mice: 35mg/kg, conception after 8 days; TDLo: 10500ug/kg, conception occurs after 10 days; abdominal TCLo of female mice: 35mg/kg, conception occurs after 12 days
Ecological data
Irritating and corrosive to skin and eyes.
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 15
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 21
8. Surface charge: 0
9. Complexity: 181
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Cannot be blended with anions.
2.It is not suitable to heat above 120℃ for a long time.
Storage method
Store in a cool, dry place and seal.
Synthesis method
1. First, cetyl alcohol and bromine are reacted under the catalysis of red phosphorus to prepare cetyl bromide, and then react with trimethylamine quaternary ammonium. .
2. Heat the cetyl alcohol and red phosphorus in a water bath with stirringMix After dissolving, add bromine dropwise, and control the temperature not to exceed 100°C. After the addition is completed, raise the temperature to 100°C to catch the hydrogen bromide gas, then cool, pour the reaction solution into water, stir until the temperature is 30~40°C, and let it stand for a while. layer, collect the oil layer, wash with 5% sodium carbonate solution until neutral, dry with anhydrous sodium sulfate, filter and distill the filtrate under reduced pressure, collect the 191-210°C fraction under 1.33MPa, which is hexadecane bromide. First, pass the trimethylamine gas generated by heating the trimethylamine aqueous solution into filtered industrial acetone to make a trimethylamine acetone solution (67g trimethylamine is absorbed per liter of acetone), and then add a mixture of hexadecane bromide and acetone (bromide Hexadecane: acetone = 1kg: 1L). After mixing evenly, slowly heat to 30°C for half an hour and then slowly heat to 40°C. Then cool the crystallization, filter it, drain it, wash the crystallization once with acetone, drain it again, and dry it at 80°C, which is the finished product.
The process reaction formula is:

4.Mainly adopt quaternization method. Using cetyl alcohol and hydrobromic acid as raw materials, in the presence of a sulfuric acid catalyst, the bromination reaction is first carried out to generate hexadecyl bromide, which is then quaternized with trimethylamine.
5.There are two synthesis methods for trimethylhexadecyl ammonium bromide. Prepare trimethylamine into an acetone solution, add hexadecane bromide dropwise under stirring, insulate and react for 1 hour, cool, crystallize, purify, spin and dry to obtain the product. The reaction formula is as follows:

It can also be produced by quaternization reaction of cetyldimethylammonium and methyl bromide. The response is as follows:
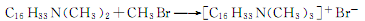
Purpose
1. Colorimetric or photometric determination of antimony and tin. Intestinal glucose absorption inhibitor. Phase transfer catalyst for aromatic hydrocarbon reduction and olefin epoxidation. Surfactant. Fungicides. Disinfectants.
2.It can be used as a bactericidal algaecide, slime stripper and cleaning agent in the water treatment industry. It has good effects on various greases in the water. Emulsification. It has good compatibility with cationic, nonionic and zwitterionic surfactants. Its antimicrobial performance is equivalent to that of geranil, and it can be used for microbial control and cleaning of circulating water systems. It has a good killing effect on heterotrophic bacteria, iron bacteria, and sulfate-reducing bacteria. Normally, its usage concentration is 50~100mg/L.
3.Cationic surfactant. Mainly used as bactericide, softener, emulsifier and antistatic agent in cosmetics. When used in hair conditioner, it can be adsorbed on the surface of the hair to form a monomolecular film, making the hair fluffy, soft and giving it a natural luster. It can also suppress the static electricity generated by friction on the hair surface, making the hair easy to comb. Since skin, hair and bacteria all have negative charges, they can be firmly adsorbed on cationic active groups to achieve moisturizing, conditioning, sterilizing and antistatic effects.
4.Used as a bactericide, disinfectant and antiseptic. It can also be used as a micellar solubilization spectrophotometric reagent. make upThe maximum allowable content in products is 0.1%, and that of deodorants is 0.05% to 0.1%.
5.Cationic surfactant, used in alkaline degreasing fluids and alkaline electroplating solutions.
extended-reading:https://www.bdmaee.net/dabco-ne1060-catalyst-dabco-ne1060-foam-catalyst-dabco-ne1060/extended-reading:https://www.cyclohexylamine.net/tetrachloroethylene-perchloroethylene-cas127-18-4/extended-reading:https://www.cyclohexylamine.net/dabco-r-8020-jeffcat-td-20-teda-a20/extended-reading:https://www.newtopchem.com/archives/817extended-reading:https://www.newtopchem.com/archives/100extended-reading:https://www.cyclohexylamine.net/metal-catalyst-heat-sensitive-metal-catalyst/extended-reading:https://www.newtopchem.com/archives/44147extended-reading:https://www.morpholine.org/pc41/extended-reading:https://www.bdmaee.net/toluene-diisocyanate-tdi-tdi-trimer/extended-reading:https://www.bdmaee.net/nt-cat-la-505-catalyst-cas10144-28-9-newtopchem/

