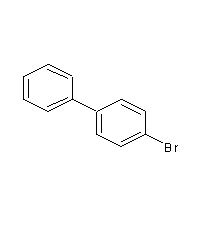
Structural formula
| Business number | 0250 |
|---|---|
| Molecular formula | C12H9Br |
| Molecular weight | 233 |
| label |
None |
Numbering system
CAS number:92-66-0
MDL number:MFCD00000100
EINECS number:202-176-6
RTECS number:DV1750100
BRN number:1907453
PubChem number:24862807
Physical property data
1. Properties: Colorless flaky crystals. Slightly aromatic smell.
2. Density (g/mL, 25/4℃): 0.9327
3. Gas phase standard entropy (J·mol-1·K-1): 440.2
4. Melting point (ºC): 191..5
5. Boiling point (ºC, normal pressure): 310
6. Gas phase standard hot melt (J·mol-1·K-1): 186.5
7. Refractive index: Not available Determined
8. Flash point (ºC): 144
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
p>
16. The logarithmic value of the oil-water (octanol/water) partition coefficient: Undetermined
17. The upper limit of explosion (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol, ether, carbon disulfide, benzene, carbon tetrachloride and acetone, slightly soluble in acetic acid, insoluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 58.53
2. Molar volume (cm3/mol): 170.9
3. Isotonic specific volume (90.2K ): 431.1
4. Surface tension (dyne/cm): 40.4
5. Polarizability (10-24cm3): 23.20
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 141
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored away from light.
Synthesis method
It is obtained from p-bromoaniline through the following reaction. Cool water, p-bromoaniline and concentrated hydrochloric acid together, keep it at 0-5°C, slowly add sodium nitrite aqueous solution, and carry out diazotization until the starch potassium iodide test paper turns blue, and filter out the clear diazo liquid. Add benzene to the diazo liquid and stir at 5-10°C for half an hour. At the same time, slowly add sodium hydroxide solution dropwise. After the addition, continue stirring for 3 hours, and then stir at room temperature for 45 hours. Separate the benzene layer, wash it with water, steam out the benzene and conduct vacuum distillation. Collect the 170-175°C (1.06kPa) fraction and recrystallize it with ethanol to obtain a finished product with a melting point of 90°C.
Purpose
Used in organic synthesis. Pharmaceutical intermediates.
extended-reading:https://www.bdmaee.net/nt-cat-9726/extended-reading:https://www.cyclohexylamine.net/trimethyl-hydroxyethyl-ethylenediamine-cas-2212-32-0/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-15.jpgextended-reading:https://www.morpholine.org/category/morpholine/page/5394/extended-reading:https://www.newtopchem.com/archives/1061extended-reading:https://www.bdmaee.net/dibutyl-tin-oxide-food-grade/extended-reading:https://www.bdmaee.net/aeea/extended-reading:https://www.newtopchem.com/archives/40418extended-reading:https://www.bdmaee.net/addocat-108/extended-reading:https://www.bdmaee.net/nt-cat-la-303-catalyst-cas1066-33-4-newtopchem/

