
Structural formula
| Business number | 017Y |
|---|---|
| Molecular formula | C4H7NO4 |
| Molecular weight | 133.1 |
| label |
L(+)-aminosuccinic acid, L(+)-aminosuccinic acid, L-aspartic acid, L-aspartic acid, (S)-(+)-Aminosuccinic acid, (S)-Aspartic acid, biochemical reagents, Intermediates |
Numbering system
CAS number:56-84-8
MDL number:MFCD00002616
EINECS number:200-291-6
RTECS number:CI9098500
BRN number:1723530
PubChem ID:None
Physical property data
1. Properties: Colorless orthorhombic leaf-shaped or rod-shaped crystals or crystalline powder, odorless. Often left-handed optical rotation.
2. Density (g/mL, 25/4℃): (d12.5/4) 1.514
3. Relative vapor density (g/mL, air=1) : Undetermined
4. Melting point (ºC): 270-271
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point ( ºC, 5.2kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific optical rotation Degree (º): [a]20/D+25° (c=1.97, in 6mol/L hydrochloric acid).
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in hot water, acid, alkali and Salt solution, insoluble in ethanol and ether.
Toxicological data
1. Acute toxicity: mouse abdominal LC50: 6mg/kg
2. Other multiple dose toxicity: rat oral TDLo: 25079mg/kg/7D-C
3. Mutagenicity: sister chromatids exchangeTEST system: human lymphocytes: 10mg/L
Ecological data
None
Molecular structure data
1. Molar refractive index: 27.20
2. Molar volume (cm3/mol): 87.8
3. Isotonic specific volume (90.2K ): 261.3
4. Surface tension (dyne/cm): 78.2
5. Polarizability (10-24cm3): 10.78
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 5
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 101
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable properties under normal temperature and pressure.
2. It is a natural product and non-toxic.
3. Exist in tobacco leaves and smoke.
Storage method
Seal and store in a dry place.
Synthesis method
1. The preparation methods of L-aspartic acid include synthesis method and fermentation method. 1. The synthesis method mainly uses maleic acid or fumaric acid or their esters as raw materials, which are treated with ammonia under pressure and then hydrolyzed. It is relatively easy to synthesize racemic aspartic acid, but so far there is no ideal method to separate the racemate. 2. In the fermentation method, fumaric acid and ammonia are added under the action of enzymes to obtain products with high yields. This method only generates the left-handed form with high yield, so it is the main method for industrial production.
2. Obtained from fumaric acid and ammonia under the action of aspartase of Pseudomonas clover or Brevibacterium ammoniagenes.
3. Use maleic acid, fumaric acid or their esters as raw materials, and add ammonia under the action of enzymes. The reaction is as follows:
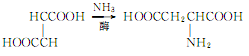
4. Tobacco: BU, 22; FC, 21.
Purpose
1. Biochemical and medical clinical research. It can be used as an ammonia detoxifier, liver function promoter, fatigue recovery agent and other pharmaceuticals. It can be used to make L-sodium aspartate food additives and additives for various refreshing drinks. It can also be used as biochemical reagents, culture media and organic synthesis intermediates.
2. Can be used as biochemical reagents, culture media and organic synthesis intermediates. In medicine, it is used as a component of heart disease drugs, liver function promoters, ammonia detoxifiers, fatigue relievers and amino acid infusions. It is also used as a preservative in the food industry.
3. Nutritional supplements. Add to various refreshing drinks. It is used medicinally as an ammonia detoxifier and liver function promoter. Used as nutritional additive in cosmetics.
4. L-aspartic acid is often used as a chiral substrate in diastereomeric alkylation reactions, and can be used as a chiral source to synthesize other chiral compounds.
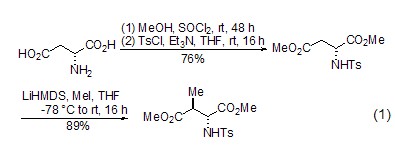
Diastereoselective alkylation L-aspartate ester can be used in α– and β– Alkylation occurs (formula 1) [2], among which the β-alkylation reaction is the most widely used. During the β-alkylation reaction, the amino acid moiety has an important influence on the diastereoselectivity of the reaction. At the same time, β-dicarbonyl compounds can also be prepared through β-alkylation of cyclic derivatives of L-aspartic acid [3].
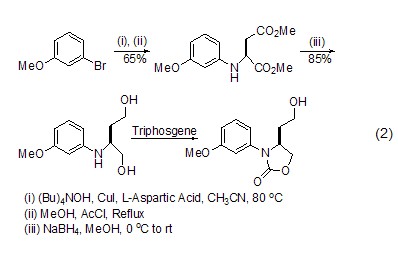
Synthesis of Chiral Compounds Using L-aspartic acid as the chiral source, a series of chiral compounds can be synthesized, such as using copper iodide After the action of sodium borohydride, etc., a multifunctional oxygen nitrogen heterocyclic compound (formula 2) can be obtained, which can further generate a quinoline compound [4].
Formation of amide bond L-aspartic acid, as an amino acid, is the same as other amino acids Amide compounds (formula 3)[5] can also be generated.
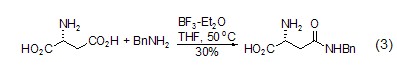
At the same time, you can also use L- Aspartic acid is used as the parent to realize the synthesis of cyclic lactam. For example, the synthesis of six-membered ring lactam (formula 4)[6]. The alkoxy group is also present in the product and therefore serves as an additional reaction site for further derivatization.
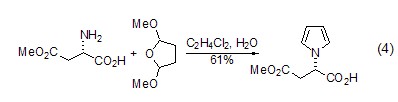
β Synthesis of amino acids (or amino acid esters) L-aspartic acid can generate β-amino acids or amino acid esters ( Formula 5)[10], this reaction realizes the conversion from natural amino acids to unnatural amino acids.
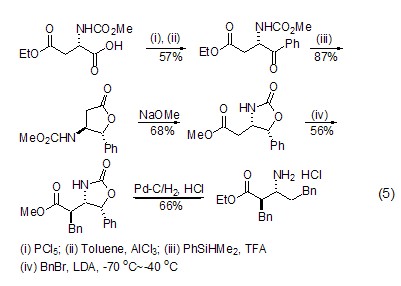
In addition, the L-aspartic acid molecule contains two carboxyl groups and one amino group, so it can be used as a multidentate ligand to coordinate with metal ions[7~9] Or form lactone compounds themselves[10,11].
extended-reading:https://www.bdmaee.net/organic-mercury-replacement-catalyst-nt-cat-e-at/extended-reading:https://www.newtopchem.com/archives/206extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/spraying-composite-amine-catalyst-NT-CAT-PT1003-PT1003.pdfextended-reading:https://www.newtopchem.com/archives/100extended-reading:https://www.newtopchem.com/archives/823extended-reading:https://www.newtopchem.com/archives/44108extended-reading:https://www.bdmaee.net/cas-6711-48-4/extended-reading:https://www.cyclohexylamine.net/k-15-catalyst-potassium-isooctanoate/extended-reading:https://www.newtopchem.com/archives/767extended-reading:https://www.newtopchem.com/archives/44131

